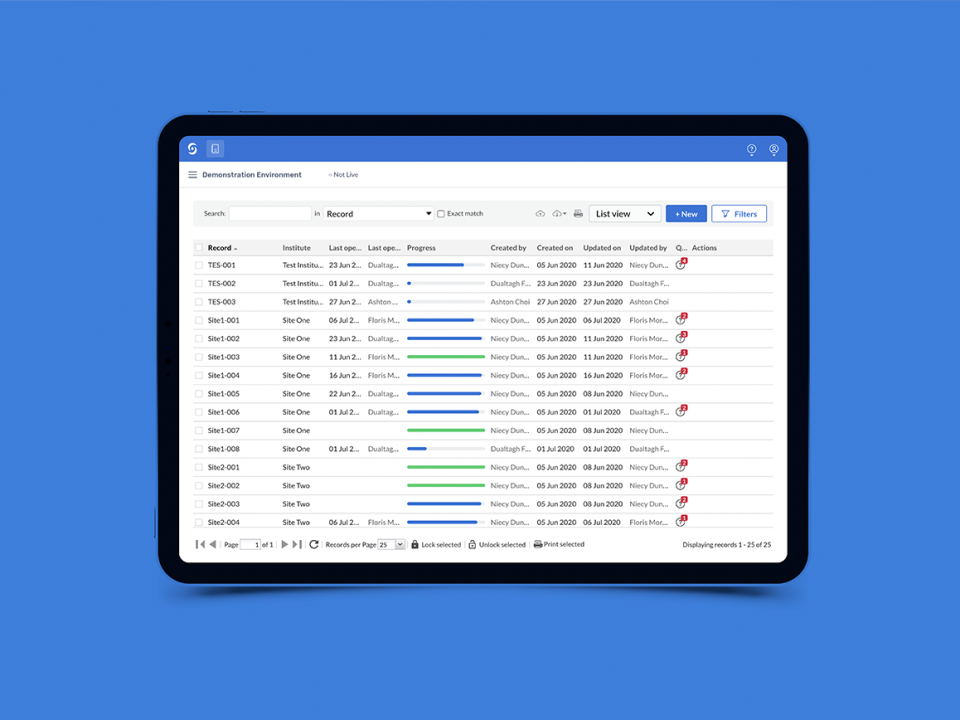
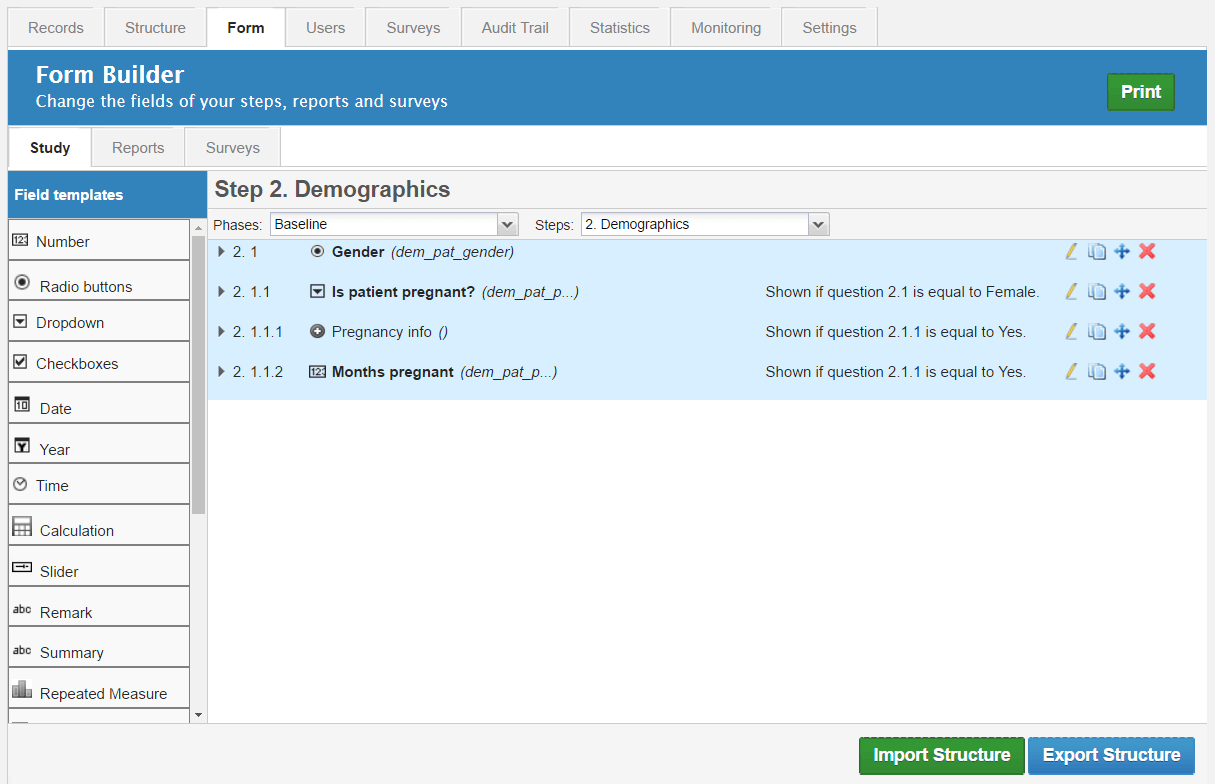
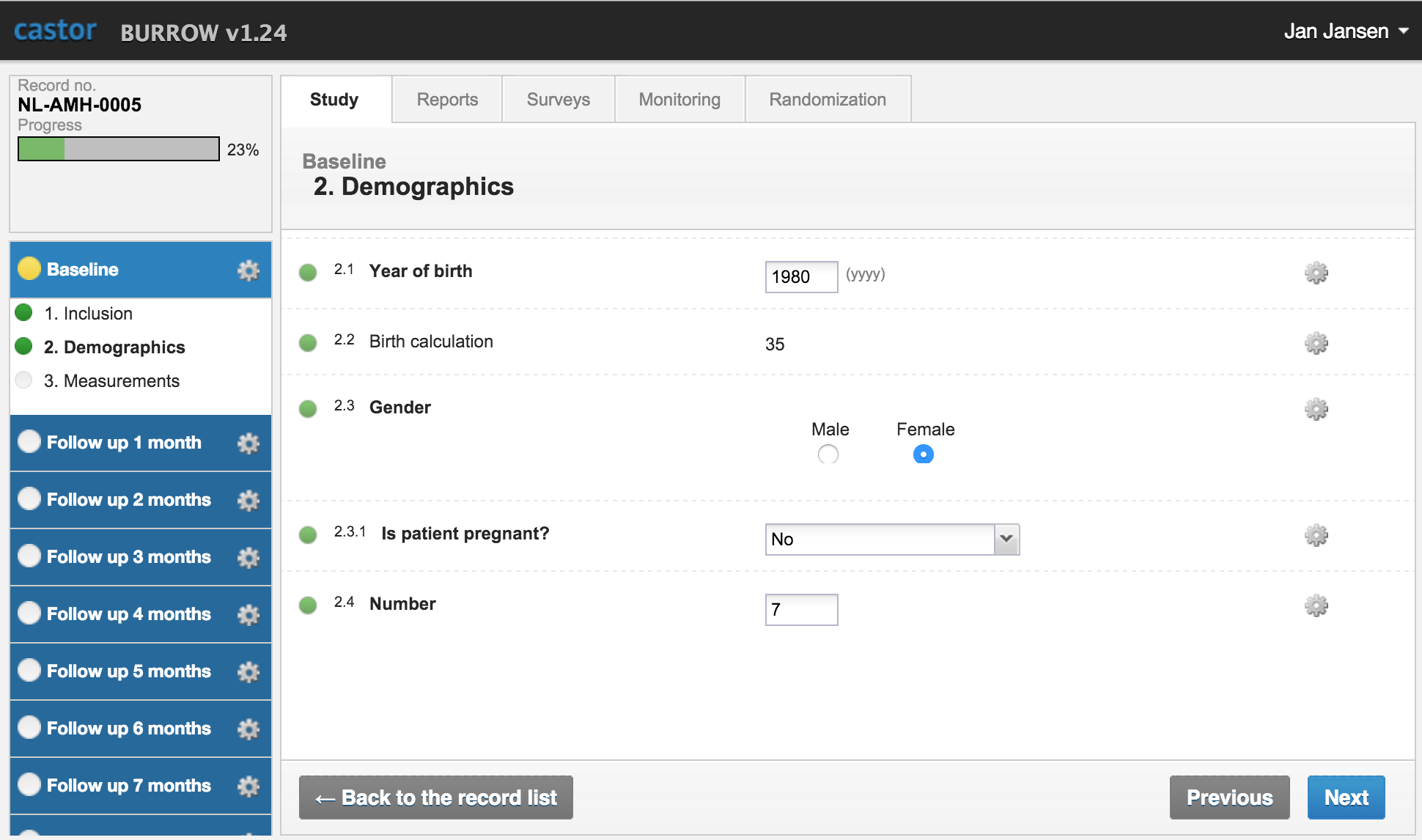
Castor Edc Login
Castor Edc Login - To be able to access the econsent platform, navigate to the login page based on the location of your study, us or europe: Welcome to the castor academy! Lost or forgot your password? Castor edc/cdms is a web application which can be accessed from anywhere using your castor login credentials. Please log in with your castor cdms account and allow the application to access your information. What you will learn from this course: Castor form exchange is a platform where you can download and upload forms and calculations that you have made in castor edc. Castor provides edc, epro, ecoa, econsent, iwrs, esource, cdms & decentralized trial solutions on a single electronic clinical data management platform. Castor edc is an electronic data capture system designed for clinical research trials. It does not offer a direct login option for users, but only links to contact support,. What you will learn from this course: It is suitable for studies that are subject to the wmo or not, and can be. In this tutorial you will be guided through the steps on how to sign up to the platform and enroll to the course. Castor edc offers a suite of modular econsent solutions to remotely recruit, screen and enroll patients in clinical trials. Lost or forgot your password? Castor edc/cdms is a web application which can be accessed from anywhere using your castor login credentials. It does not offer a direct login option for users, but only links to contact support,. This web page provides information about castor edc, a platform for electronic data capture in research. Learn how to schedule a demo, integrate with other systems, and choose the right service model for your. Castor offers edc, econsent, epro, dct, and rwe solutions for clinical research. To access the forms, you need to log in with your castor. All you need to know to add and customize. Castor edc offers a suite of modular econsent solutions to remotely recruit, screen and enroll patients in clinical trials. Castor edc is an electronic data capture system designed for clinical research trials. Castor offers electronic data capture (edc) tools. Learn how to schedule a demo, integrate with other systems, and choose the right service model for your. Please log in with your castor cdms account and allow the application to access your information. Lost or forgot your password? Learn how to use video visits, patient portals, icf management and. For the best experience, we recommend using the latest version. To access the forms, you need to log in with your castor. All you need to know to add and customize. For the best experience, we recommend using the latest version of google. Learn how to build advanced ecrfs, integrate data from various sources,. Castor offers edc, econsent, epro, dct, and rwe solutions for clinical research. For the best experience, we recommend using the latest version of google. Castor edc/cdms is a web application which can be accessed from anywhere using your castor login credentials. Castor provides edc, epro, ecoa, econsent, iwrs, esource, cdms & decentralized trial solutions on a single electronic clinical data management platform. It is suitable for studies that are subject to the. Collecting high quality data is critical for any research. In this tutorial you will be guided through the steps on how to sign up to the platform and enroll to the course. Castor edc is an electronic data capture system designed for clinical research trials. All you need to know to add and customize. Castor edc offers a suite of. Castor provides edc, epro, ecoa, econsent, iwrs, esource, cdms & decentralized trial solutions on a single electronic clinical data management platform. What you will learn from this course: Castor edc/cdms is a web application which can be accessed from anywhere using your castor login credentials. It does not offer a direct login option for users, but only links to contact. All you need to know to add and customize. Learn how to build advanced ecrfs, integrate data from various sources,. It does not offer a direct login option for users, but only links to contact support,. Lost or forgot your password? Castor edc offers a suite of modular econsent solutions to remotely recruit, screen and enroll patients in clinical trials. To access the forms, you need to log in with your castor. Castor offers edc, econsent, epro, dct, and rwe solutions for clinical research. To be able to access the econsent platform, navigate to the login page based on the location of your study, us or europe: Please log in with your castor cdms account and allow the application to. Castor edc/cdms is a web application which can be accessed from anywhere using your castor login credentials. Collecting high quality data is critical for any research. Please log in with your castor cdms account and allow the application to access your information. Castor provides edc, epro, ecoa, econsent, iwrs, esource, cdms & decentralized trial solutions on a single electronic clinical. Lost or forgot your password? Castor edc offers a suite of modular econsent solutions to remotely recruit, screen and enroll patients in clinical trials. Castor offers electronic data capture (edc) tools created by researchers, for researchers. You need to be a registered castor edc user in order to access this page. Learn how to build advanced ecrfs, integrate data from. You need to be a registered castor edc user in order to access this page. Collecting high quality data is critical for any research. Learn how to build advanced ecrfs, integrate data from various sources,. Castor edc/cdms is a web application which can be accessed from anywhere using your castor login credentials. Learn how to schedule a demo, integrate with other systems, and choose the right service model for your. Castor offers edc, econsent, epro, dct, and rwe solutions for clinical research. It is suitable for studies that are subject to the wmo or not, and can be. Please log in with your castor cdms account and allow the application to access your information. Castor edc is an electronic data capture system designed for clinical research trials. It does not offer a direct login option for users, but only links to contact support,. To access the forms, you need to log in with your castor. Learn how to use video visits, patient portals, icf management and. What you will learn from this course: To access castor, enter your email address and. Castor edc offers a suite of modular econsent solutions to remotely recruit, screen and enroll patients in clinical trials. All you need to know to add and customize.Introducing the new and improved Castor EDC Castor
Castor EDC Software Reviews, Demo & Pricing 2024
Electronic Data Capture Software 5 Apps for Clinical Research
Castor EDC Reviews and Pricing 2018
Features Castor EDC Castor EDC
Castor EDC Alternatives and Similar Software
Castor EDC precios, funciones y opiniones GetApp España 2021
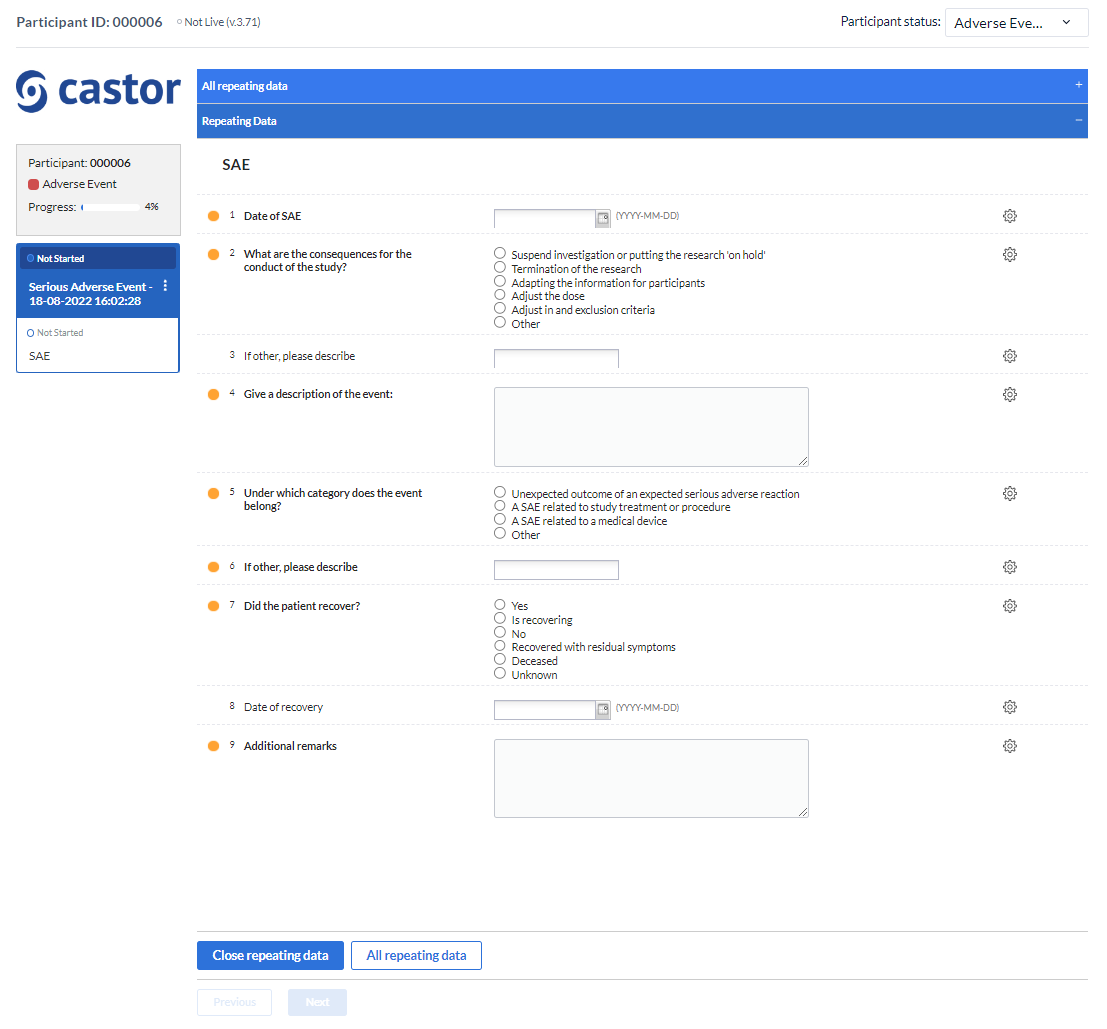
Serious Adverse Events (SAE) EDC/CDMS Castor
Features List Castor EDC
GitHub castoredc/castoRedc R wrapper to Castor EDC API
To Be Able To Access The Econsent Platform, Navigate To The Login Page Based On The Location Of Your Study, Us Or Europe:
Castor Provides Edc, Epro, Ecoa, Econsent, Iwrs, Esource, Cdms & Decentralized Trial Solutions On A Single Electronic Clinical Data Management Platform.
In This Tutorial You Will Be Guided Through The Steps On How To Sign Up To The Platform And Enroll To The Course.
For The Best Experience, We Recommend Using The Latest Version Of Google.
Related Post: